Buffer In Chemistry Definition
Buffer In Chemistry Definition. Its ph changes very little when a small amount of. Examples of buffer in the following topics:
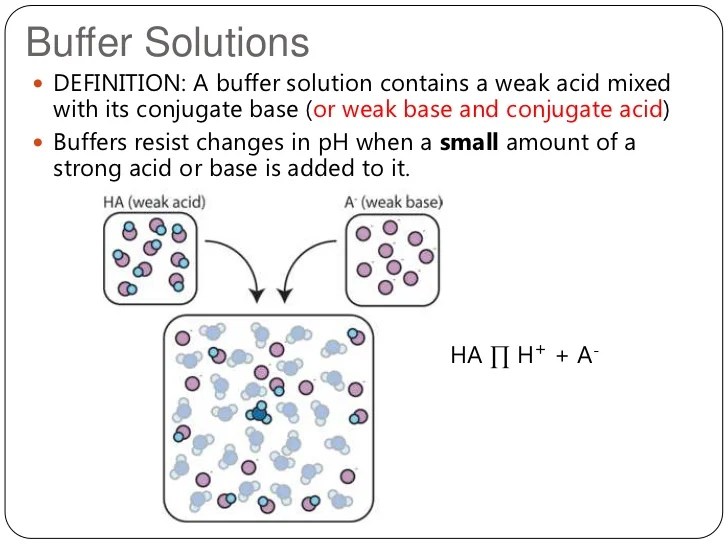
Buffers are solutions that can resist changes in ph when small amounts of acids or bases are added to them. A buffer's capacity is the ph range where it works as an effective buffer, preventing large changes in ph upon addition. Its ph changes very little when a small amount of.
It Is Able To Keep The Solution’s Ph Stable By Taking Small Amounts Of Acid And Base.
A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base.
Let's Begin By Defining What Buffers Are.
A buffer is a concentrated solution of a weak base and its conjugate acid. A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding. Buffer solution one that resists appreciable change in its hydrogen ion concentration (ph) when acid or alkali is added to it.
Its Ph Changes Very Little When A Small Amount Of.
Specific and stable ph ranges are important for. Colloid solution ( colloidal solution ) imprecise term for colloid (def. A buffer is an aqueous solution that has a highly stable ph.
A Solution Which Can Maintain An Almost.
It consists of a solution of a weak acid and its conjugate. A buffer's capacity is the ph range where it works as an effective buffer, preventing large changes in ph upon addition. A buffer solution refers to an aqueous solution.
Buffers Are Solutions That Can Resist Changes In Ph When Small Amounts Of Acids Or Bases Are Added To Them.
Examples of buffer in the following topics: What is buffer in chemistry? Ions are atoms or molecules that.
Post a Comment for "Buffer In Chemistry Definition"